Type 2 diabetes variants disrupt function of SLC16A11 through two distinct mechanisms
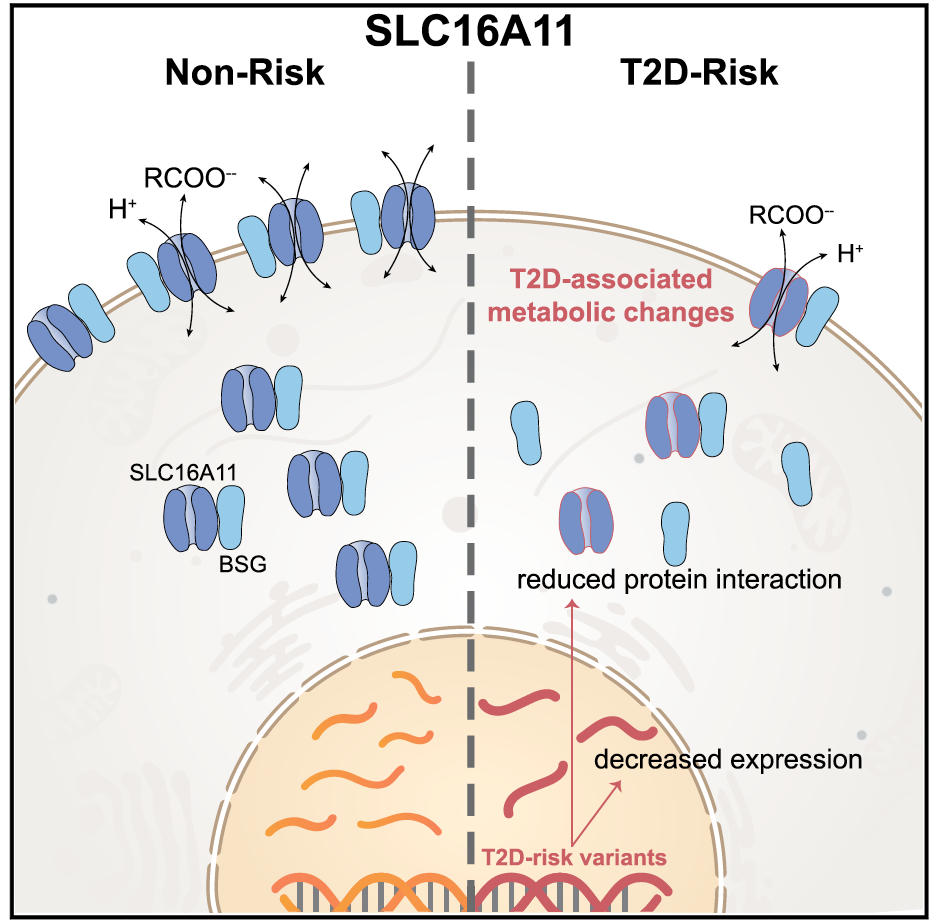
Type 2 diabetes (T2D) affects Latinos at twice the rate seen in populations of European descent. We recently identified a risk haplotype spanning SLC16A11 that explains ?20% of the increased T2D prevalence in Mexico. Here, through genetic fine-mapping, we define a set of tightly linked variants likely to contain the causal allele(s). We show that variants on the T2D-associated haplotype have two distinct effects: (1) decreasing SLC16A11 expression in liver and (2) disrupting a key interaction with basigin, thereby reducing cell-surface localization. Both independent mechanisms reduce SLC16A11 function and suggest SLC16A11 is the causal gene at this locus. To gain insight into how SLC16A11 disruption impacts T2D risk, we demonstrate that SLC16A11 is a proton-coupled monocarboxylate transporter and that genetic perturbation of SLC16A11 induces changes in fatty acid and lipid metabolism that are associated with increased T2D risk. Our findings suggest that increasing SLC16A11 function could be therapeutically beneficial for T2D. VIDEO ABSTRACT here.
PIIS0092867417306943.pdf




